|
|
|
Autre version : CHIME version
What is cantharidin ?
The cantharidin was first isolated by Robiquet, a French chemist in 1810. It has an important role in the ecology of different kinds of insects that use or produce it as a defense ability to preserve their eggs from predators [1]. In the ancient world, the dried spanish flies had the reputation of aphrodisiac virtues [27]. In fact, these supposed properties are not attested neither in theory nor in experimentation. The cantharidin seems to be a dangerous substance with the same toxicity as the most violent poisonus like strychnin.|
|
|
The cantharidin molecule
At the usual temperature, the cantharidin is an odorless and colorless solid. Its formula is C10H12O4 and its melting point is 218 °C.
|
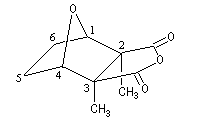 The cantharidin or 2, 3-dimethyl-7-oxabicyclo[2, 2, 1]heptane-2, 3-dicarboxylic anhydrid represented on the picture shown above, is an achiral compound. It has a plane of symmetry that goes through the middles of the bonds C2C3 and C5C6. It's a meso compound (2S, 3R).
|
There is an other molecule with a similar structure : the palasonin. This molecule is produced by a tree butea frondosa that grows in the Himalaya. Contrary to the cantharidin, the palasonin is chiral and exists with two enantiomeric forms. The natural compound represented below is (-)-palasonin.
|
The two enantiomeric forms of the palasonin. Only the left exists in the nature.
|
Biosynthesis
The farnesol represented on the picture is the (E, E)-3, 7, 11-triméthyldodeca-2, 6, 10-triene-1-ol. This is a sesquiterpenic alcohol. It is an intermediate in the biosynthesis of isoprenoïds.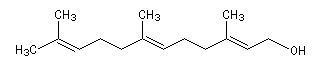 The farnesol is quite abundant in the nature. A lot of natural essences, like Ylang-ylang ones, contain this molecule.
|
The following diagram shows some cleavages and insertion of oxygen in a specific conformation of the farnesol that will lead to the cantharidin [20].
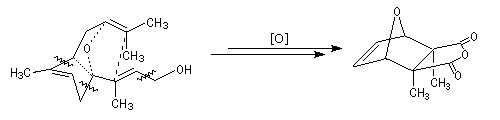
Mode of action of the cantharidin
First attemps for the synthesis of the cantharidin
Von Bruchhausen, a german chimist, first attempted the synthesis of the cantharidin [2]. It was based on the following retrosynthetic analysis.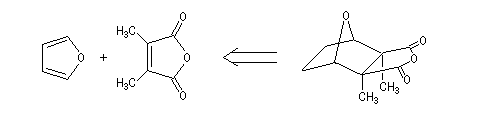
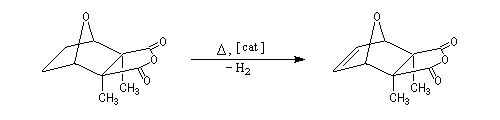
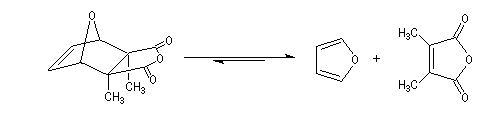
A more deepened study shows that the instability of the product is due to the repulsion between the methyl groups carried by C2 and C3 and also to the repulsion between the hydrogen carried by C5 et C6 (the covering between the Van der Waals spheres is visible with the "spacefill" representation of the molecular model of the cantharidin).
Synthesis of the cantharidin
The Stork synthesis
The first effective synthesis of the cantharidin was made in 1951 by the American chemist G. Stork (born in Belgium) [4] and [5]. The synthesis is completly linear and only uses classical reactions. It's a typical total synthesis of natural product dating from this period.
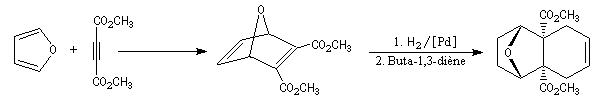
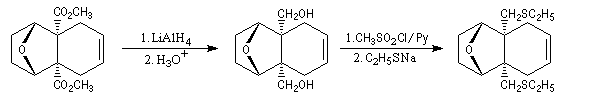
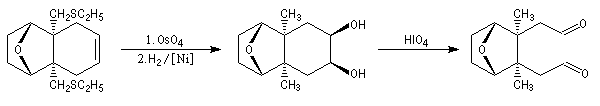
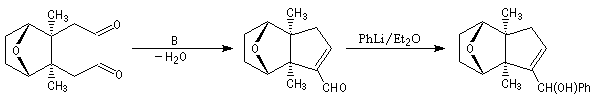
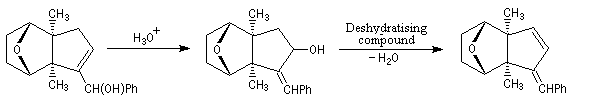
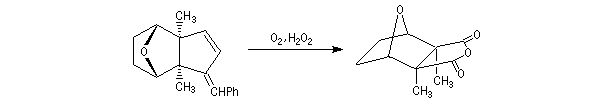
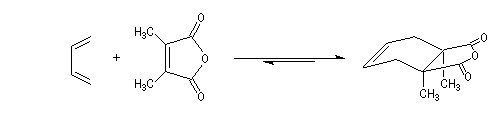
The Schenk synthesis
The Schenk's synthesis first uses a Diels-Alder reaction but here, the repulsions between the methyl groups don't exist and the equilibrium is favourable to the product [6].
Conjugated double bonds are made with the addition of Br2 on the ethylenic bond following by a double elimination with DBU.
DBU means 1,8-diazabicyclo[5.4.0]undec-7-ene. It's a bulky base that permits elimination.
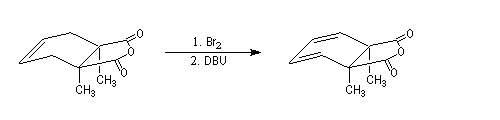
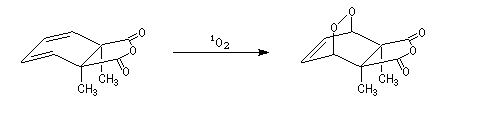
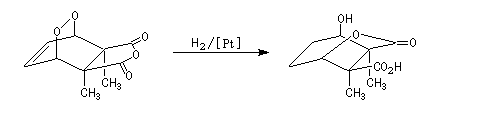
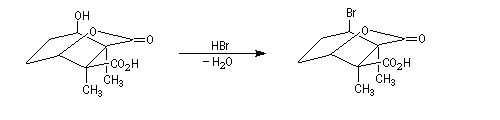
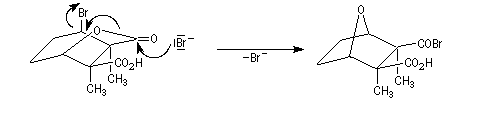
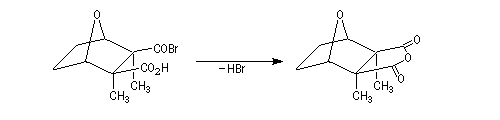
The Dauben's synthesis
Dauben is an American chemist in Berkeley University. He uses high pressure to realize a lot of synthesis. The Diels-Alder's reaction is realizable
thanks to a pressure of 15 kbar. And then the synthesis of cantharidin necessitates only two steps [7], [8].
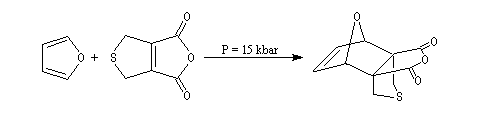
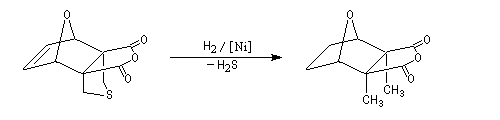
Bibliography
Links
For educational purpose - No commercial use
Page created by Gérard Dupuis - Lycée Faidherbe LILLE
gdupuis-prof@faidherbe.org
This site was last updated January 2004