|
|
Oscillating reactions - Chemical waves
by G. Dupuis and N. Berland
|
|
|
Oscillating reactions - Chemical waves
by G. Dupuis and N. Berland
|
Also available : french version
What is an oscillating reaction ?
A macroscopic reaction can be explained by a mechanism consisting of a great deal of elementary reactions in the microscopic scale. In these reactions, or steps, some species are created and then consumed. In most chemical reactions, the concentrations of species depend monotonously on the time but in
an oscillating reaction, the concentrations of some species increase and then decrease during the time, between two limits. Some properties like colour, absorption or average potential can change during the experiment with a more or less periodical evolution.
Due to these facts, some of these oscillating reactions are used as chemical clocks.
Experimental aspect
The following reaction is the Belousov-Zhabotinsky reaction. It isn't difficult. We begin to prepare 5 aqueous solutions with concentration in units of mol.L-1. Then, we mix these solutions in a big beaker, increase the temperature around 25 °C and stir well. The oscillations appear after some seconds [11], [12].
|
KBrO 3 |
Ce(NH4)(NO3)5 |
CH2(COOH)2 |
H2 SO4 |
[Fe(oPhen)3 ] |
|
6,25 10-2 |
2,0 10-3 |
2,8 10-1 |
1,5 |
6,0 10-4 |
![]()
|
The opposite animation gives an approximative idea of the phenomenon observed during the Belousov-Zhabotinsky reaction. The colour changes from red to blue. Whithout indicator, the colour changes from yellow to colourless. The manipulation must be done under an efficient hood. Br2 appearing at the end of the reaction. |
![]()
The electrodes are linked at the analogic entering of a PC-MES 2 card (Eurosmart) and the results are treated by SYNCHRONIE program.

The graph, giving the average potential (pot) during the time is representated below :
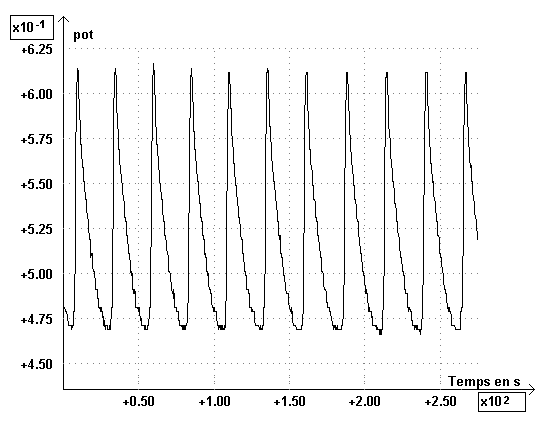
The Fourier analysis of the graph can be obtained by SYNCHRONIE program, and we observe one fundamental frequency, showing the periodicity of the phenomenon. The presence of harmonics is due to the non sinusoïdal nature of signal. We have realized a chemical clock.

The BZ reaction mechanism
The complete mechanism of the BZ reaction was made by R. M. Noyes, R. J. Fields and E. Körös. It counts 18 réactions with 21 différent chemical species. A simplified mechanism was proposed by the same authors. It was named "Oregonator" in reference to the University of Oregon
where they worked in this period. The different steps of this mechanism are done below :
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Kinetic models
A lot of kinetic models were proposed to describe the BZ reaction. To simplify the equations we'll use the following notations :
|
Compound |
BrO 3- |
Orga |
HBrO |
HBrO 2 |
Br- |
Ce4+ |
|
Notation |
A |
B |
P |
X |
Y |
Z |
Then, the simplified mechanism of FKN, limited to the steps (a), (d'), (e), (f) and (h) is :
This system isn't linear. Some results can be obtained by numerical methods. We observe oscillating results for f values from 0,5 to 2,4.
Chaotic system
The seventies developped some important works about non linear phenomenons. One of the most great conclusion of these studies is that some dynamic systems belonging to various parts of physic
have unexpected behaviour although they are described by determinist rules. These systems have a large sensitivity to the initial conditions, and are named "determinist chaos". It's logical to wonder if the BZ reaction, which dynamic behaviour is described by non linear equations, can have a chaotic behaviour.
Some experiments, done by Schmitz and Hudson in 1977, showed the appearance of chaos in the BZ reaction. And then, the BZ reaction permitted to test some theoretical aspects of dynamic systems [5], [24].
Diffusion phenomenon
Put gently a drop of ink in a beaker full of water. The ink ends up invading all the solution.
When a solution is quiet with a uniform temperature, the convection phenomenon can be neglected. In these conditions, it's the diffusion which provides the transport of compounds.
A spontaneous transfer appears from the zones of high concentrations to these ones of lower concentrations. With self-catalytic reactions like BZ reaction, the situation is completly different. The coupling between chemical reaction and diffusion can create
organizations in space. We can observe two phenomenons of organization :
Chemical waves
The discovery of chemical waves was due to A. Zhabotinsky [33]. The first systematic works about the BZ reaction were published by A. Winfree en 1972. The experiment below, permits to show the propagation of chemical waves during the BZ reaction, in a thin rest coat. The experiment is quite the same as this one proposed by J. -C. Micheau in [14]. Prepare three solutions at around 20 °C :
|
|
|
|
|
I |
II |
III |
|
|
|
The BZ reaction with malonic acid as an initial substrate, has the inconvenient to create a gas : CO2. Several possibilities were explored to develop gas-free version of the BZ reaction. In one of this reaction malonic acid is replaced by 1,4-cyclohexanedione or 1-3-cyclohexanedione (CHD) as an initial substrate. The system is : CHD/BrO3-/Ce4+/H+.
|
|
Thanks to Professor A. M. Zhabotinsky (Brandeis University Waltham) who gave us the permission of published this picture [31]. |
In 1993, Anatol M. Zhabotinsky and his colleagues demonstrated experimentally reflection and refraction of chemical waves using a reaction in a gel. Reaction-diffusion systems are non linear so it's surprising for chemical waves to follow Snell's law. By solving the reaction-diffusion equations, R. Dilao and J. Sainhas of the instituto Superior Técnico Lisboa, Portugal proved mathematically that chemical waves obey Snell's law [40], [6].
The Turing's structures
The english mathematician Alan Turing, tried to establish a theory of morphogenesis. In an item published in 1952, called : The chemical basis of morphogenesis [15], he showed how a chemical reaction
coupled with a phenomenon of diffusion, could deal to spatial periodic distributions of the concentrations of some chemical compounds. The observation of the Turing's structure needs some experimental conditions :
all the phenomenons of convection must be avoided and then the transportation of compounds must be due to the diffusion only. The reaction is sometimes made in a gel [19].
In 1989, the P de Kepper group tried to visualize I-, in the CIMA (chlorite, iodide, and malonic acid).
In a gel, after the addition of starch (an indicator), these research workers saw some regular rows of task in the reactor as Turing had predicted them. The theoretical calculations in the thermodynamic of irreversible process, show that the phenomenon
appears only if the compounds have very different coefficients of diffusion [1]. The starch is a big molecule which diffuse slower than I- in the gel and this difference
permits the observation of the Turing's structure [7].
|
|
Thanks to Dr. J. Boissonade (CRPP Bordeaux) who gave us the permisson of published this picture [19]. |
Some thermodynamics developments
The Laws of thermodynamics can describe the behaviour in the macroscopic scale of systems constituted of a great deal of microparticles.dU = dQ + dW
Where dQ is the exchange of energy as heat and dW the exchange of energy as work. In an isolated system :dU = 0
U is constant. The First Law is the conservation of energy in an isolated system.
If the system isn't isolated, U evoluates. For thermomechanical transformation where Pe is the pressure of the environment :
dW = - Pe.dV
If the mechanical equilibrium is established between the system and its environment : Pe = PdU = dQ - P.dV
if P is constant :
dQ = dHP
where :H = U + P.V
H is a state function called enthalpy (H for heat). The determination of dQ is possible if the initial and final states are known.dS = diS + deS
deS = dQ/TE
if a system doesn't exchange heat :
deS = 0
Even if it exchanges energy by works. So this principle establishes a difference between Q and W.
|
diS = 0 |
diS > 0 |
|
Equilibrium |
Spontaneous evolution |
The entropy isn't a conservative function like U.
Reversible transformation
Reversible transformations are ideal and we can describe them by a succession of equilibrium states between the system and its environment during the evolution from the initial state
to the final state and we can reverse the sense of time and then recome from the final state to the initial one.
In this ideal case :
diS = 0
Spontaneous transformation
For these transformations :
diS > 0
It's difficult to use this inegality. But the entropy is a function whose variation depends only on the initial state A and final state B. And then we can calculate the variation of S with another reversible transformation which would lead the system from A to B.Chemical affinity
When there is a chemical reaction in a system, the entropy can be considered like a function of the energy U, the volume V and the quantities of material, nk. In a closed system, the different nk are linked by the stoichiometry of the reaction. Their variations can be expressed with only one variable : the advancement x.
S = S (U, V, x)
The Belgian chemist de Donder, introduced a new notion : the affinity A (1922) [1], [16], linked to diS by the relation :
diS = (A/T).dx
where T is the temperature of the system. The rate of reaction v is :
v = dx/dt
And then :
diS/dt = (A/T).v
The production of entropy per units of time appears as a multiplication of 2 terms :
We can observe two spontaneous transformation where diS > 0 splitted by the chemical equilibrium where diS = 0 :
|
A < 0 |
A = 0 |
A > 0 |
|
dx/dt < 0 |
dx/dt = 0 |
dx/dt > 0 |
diS/dt = S (An/T).vn
This one permits to distinguich three thermodynamical estates which, as I. Prigogine wrote in La nouvelle alliance [4], correspond to the three steps of the thermodynamical evolution.G = H - T.S
In Europe we call this function the free enthalpy because in some conditions G represents the enthalpy which can be converted in works. Let us calculate the differential of G when T and P are constant.
dGT,P = dU + P.dV - T.dS
For a thermomechanical transformation :
dU = - P.dV + dQ
We obtain :(or) :
dGT,P = - A.dx
(¶G/¶x)T, P = - A
the evolution is spontaneous with a positive rate of reaction if :
(¶G/¶x)T, P < 0
the equilibrium is obtained when :
(¶G/¶x)T, P = 0
G is a potential function. G decreases in a spontaneous reaction until a minimal value when the equilibrium is obtained.
If, when the system is in an equilibrium state, a small perturbation removes the system from this state, x varies of dx and then, the disrupted system evoluates. The irreversible phenomenons will bring back the system in its initial state. And so, when a system is in an equilibrium state, he can't leave it spontanously. The stability of an equilibrium state face to face fluctuations, explains the impossibility to observe oscillations of concentrations near this state. But if the system is far from equilibrium, it's able to observe these oscillations.
dx/dt = m.x - x2
m is a parameter which can have various real values.
|
|
dx/dt = m.x - x2
|
[1] I. Prigogine, D. Kondepudi - Thermodynamique, Editions Odile Jacob, 1999.
[2] A.Pacault, J.J. Perraud - Rythmes et formes en chimie, P.U.F, 1997.
[3] C. Vidal, G. Dewel et P. Borckmans - Au-delà de l'équilibre, coll. Enseignement des sciences, Hermann, 1994.
[4] I. Prigogine, I. Stengers - La nouvelle alliance, Gallimard, 1986.
[5] C. Vidal, H. Lemarchand - La réaction créatrice : dynamique des systèmes chimiques, Hermann, 1988.
[6] Belousov-Zhabotinsky reaction by G. Dupuis and N. Berland, Encyclopedia of Nonlinear Science NL 3600, ed. Alwyn Scott, Taylor and Francis New-York (2004).
[7] P. De Kepper et al., Taches, rayures et labyrinthes, La recherche n° 305, janvier 1998.
[8] R. M. Noyes, Mechanisms of Some Chemical Oscillators, J. Phys. Chem., 94, 4404, 1990.
[9] I. Epstein, K. Kustin, P. de Kepper, M. Orban - Réactions oscillantes, Pour la science, 1983.
[10] C. Vidal, La réaction chimique créatrice de rythme et de formes, Bulletin de l'Union des Physiciens, mars 1992.
[11] J.,-F. Lefelhocz, J. Chem. Ed. 49, 312 (1972).
[12] J. L. Brisset, Systèmes chimiques oscillants, Bulletin de l'Union des Physiciens, décembre 1980.
[13] V. Pimienta, D. Lavabre, J. C. Micheau, G. Levy, Les systèmes chimiques hors équilibre : exemples de bistabilité et d'oscillations, Bulletin de l'Union des Physiciens, février 1999.
[14] J. C. Micheau, Oscillations chimiques et structures spatiales, Bulletin de l'Union des Physiciens, mars 1992.
[15] A. Turing, The chemical basis of morphogenesis, Philos. Trans. Roy. Soc. London, B 237, 37, 1952.
[16] H. Gié, l'affinité chimique, Bulletin de l'Union des Physiciens, octobre 1968.
[32] The bromate-1,4- cyclohexanedione-ferroin gas-free oscillating reaction Kurin-Csörgei, K., Zhabotinsky, A. M., Orbán, M. and Epstein, I. R., J. Phys. Chem. 100, 5393 (1996).
[33] Concentration wave propagation in two-dimensional liquid phase self oscillating system A. N. Zaikin and A. M. Zhabotinsky, Nature, 225, 535-537 (1970).
[34] Periodical oxidation of malonic acid in solution (a study of the Belousov reaction kinetics), A. M. Zhabotinsky Biofizika, 9, 306-311 (1964).
[35] A. T. Winfree, Science 1973, 181, 937.
[36] R. J. Fields, E. Körös, R. L. Noyes, J. Am. Chem. Soc, 1972, 94, 8649.
[17] Circadian Rhythms and Oscillating reactions by James Baird - Brown University
[18] The BZ reaction by Gabriel Peterson - College of the Redwoods
[19] Morphogénèse chimique - Centre de recherche P. Pascal
[20] Internet Differential Equation Activities - Washington State University
[21] The phenomenology of the Réaction BZ by Peter Ruoff
[22] Des réactions oscillantes à la formation des motifs chimiques - Département de chimie de l'Ecole
Normale Supérieure
[23] Cyberlabo
[24] Portrait de phase de la réaction BZ
[25] Réactions oscillantes
[26] Rythmes et formes en chimie
[27] I. Prigogine - Fondation Nobel
[28] Quadratic iteration, bifurcation and chaos
[29] Oscillating Reactions Web Module University of Michigan
[30] Oscillating reactions Dartmouth College
[31] Page de A. M. Zhabotinsky, Brandeis University Waltham
[40] Refraction of chemical waves
[41] An Introduction to Nonlinear Chemical Dynamics (by I. R. Epstein and J. A. Pojman) JCE April 2000 Reviewed by R. J. Field
[42] Non Linear Science FAQ
[43] Morphogenesis: an interdisciplinary scientific field
[44] Encyclopedia of non linear science
For educational purpose - No commercial use
Page created by Gérard Dupuis - Lycée Faidherbe LILLE
gdupuis-prof@faidherbe.org
This site was last updated November 2004